| Jump to | Jump to | Jump to |
In vitro studies of osteoblast-adipocyte interactions:
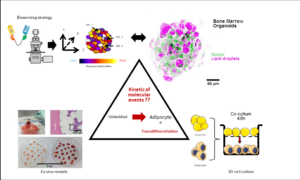
The research project of the team focuses on the involvement of medullary adipocytes in the bone loss of age-related osteoporosis through their negative impact on neighboring osteoblasts. In an attempt to reproduce cellular interactions within the bone marrow, we developed cellular models using adipocytes and osteoblasts derived from human bone marrow stromal cells or from bone biopsies. In these models, it was demonstrated that soluble factors secreted by adipocytes induced the conversion of osteoblasts towards an adipocyte-like phenotype, providing evidence for a transdifferentiation event. In this context, one of our aim is to identify adipocyte-secreted factors and molecular mechanisms responsible for the phenotypic conversion of osteoblasts. A bioinformatic approach connecting proteomic and transcriptomic data led to the identification of three signaling pathways that may promote osteoblast transdifferentiation. The assessment of such complex biological processes cannot be achieved using conventional methods, and requires the support of quantitative microscopy approaches relying on functional live cell imaging to study the spatio-temporal relationships between identified pathways. Another aim is to validate the implication of this phenomenon in 3D models, such as vascularized bone marrow organoids which recreate the complexity of the environmental niche of bone tissue.
Areas of Expertise
- Cellular models
- Human bone marrow cells
- Cell signaling
- Quantitative microscopy methods
Technology Transfer Potential
Organoid applications in osteopathology: drug screening, personalized medicine, regenerative medicine.
Bibliography
- Salmi A, Quacquarelli F, Chauveau C, Clabaut A, Broux O. An integrative bioinformatics approach to decipher adipocyte-induced transdifferentiation of osteoblast. Genomics, 2022 Jul;114(4):110422.
- Clabaut A, Grare C, Rolland-Valognes G, Letarouilly JG, Bourrier C, Andersen TL, Sikjær T, Rejnmark L, Ejersted C, Pastoureau P, Hardouin P, Sabatini M, Broux O. Adipocyte-induced transdifferentiation of osteoblasts and its potential role in age-related bone loss. PLoS ONE, 2021;16(1). e0245014.
- Roebroek T, Vandenberg W, Sipieter F, Hugelier S, Stove C, Zhang J, Dedecker P. Simultaneous readout of multiple FRET pairs using photochromism. Nat Commun., 2021; 12(1):2005.
- Letarouilly JG, Broux O, Clabaut A. New insights into the epigenetics of osteoporosis. Genomics, 2019 Jul;111(4):793-798.
- Clabaut A, Broux O. Omics Contributions to the Molecular Mechanisms Regulating Bone Marrow Adipocyte Differentiation. Current Molecular Biology Reports, 2018, 4 (1): 1–7.
- Clabaut A, Grare C, Léger T, Hardouin P, Broux O. Variations of secretome profiles according to conditioned medium preparation. Electrophoresis, 2015, 20:2587-93.
- Martin PJ, Haren N, Ghali O, Clabaut A, Chauveau C, Hardouin P, Broux O. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC Cell Biol., 2015, 16:10.
- Lucas S, Clabaut A, Ghali O, Haren N, Hardouin P, Broux O. Implication of fatty acids in the inhibitory effect of human adipocytes on osteoblastic differentiation. Bone, 2013, 55(2):429-30.
- Hardouin P, Biver E, Pansini V, Clabaut A, Broux O, Delplace S, et al. Are bone marrow adipocytes involved in pathology? Osteoporosis Int., 2013;24(SUPPL.3):S450-S453+S473.
- Clabaut A, Delplace S, Chauveau C, Hardouin P, Broux O. Human osteoblasts derived from mesenchymal stem cells express adipogenic markers upon coculture with bone marrow adipocytes. Differentiation, 2010, 80:40-45.
Projects
Doctoral contract for a joint thesis (ULCO / Università Politecnica della Marche), 2019-2023. “Medullary adipocytes and age-related osteoporosis”. VINCI 2019 program, Université Franco-Italienne (UFI).
Programme de recherche STaRS (Soutien à l’accueil de Talents de la Recherche Scientifique, Chercheurs Permanents de Haut Niveau) Région Hauts de France, 2023-2025. ADIPOSCELL3D : Culture 3D d’organoides, biosenseurs et optogénétique, pour mieux comprendre les mécanismes médullaires de l’ostéoporose.
CPER – MOSOPS, 2024-2026. BIOREOS : Biomatériaux organoides pour la reconstruction osseuse.
Cellular models and tools developed to identify molecular mechanisms of osteoblast to adipocyte transdifferentiation.
Team members
Odile Broux , research engineer; Aline Clabaut, assistant professor; Frederic Degardin, associate professor; Damien Leterme, assistant professor ; Véronique Gauthier, assistant engineer; François Sipieter, assistant professor; Diara Thiane, study engineer.
Clinical approach of BMAT
The ADIMOS study was conducted to evaluate bone marrow adiposity as a biomarker of fracture risk in postmenopausal women with osteoporosis. The study included 200 postmenopausal women, half of whom had recently experienced a fracture and the other half had no history of fragility fractures.
The laboratory demonstrated its ability to assess bone marrow adiposity using MRI on two sites (proximal hip and lumbar spine) with two different techniques (Dixon and spectro-MRI). The results showed that measuring bone marrow adiposity at the proximal hip can discriminate between patients with or without vertebral fractures, independently of other classic risk factors for osteoporosis.
Team members
Pr. Julien Paccou (Rheumatologist), Sammy Badr (Radiologist), Pr. Anne Cotten (Radiologist), Pr. Bernard Cortet (Rheumatologist)
In-vivo approach of BMAT
We studied the role of the local environment in bone loss in animal model. We evaluated the intra-osseous lipid signature as lipids play an important role in bone physiology. The increase in the activity of the enzyme ∆9-desaturase, measured by the ratios 16:1/16:0 and 18:1/18:0, is associated with bone loss related to osteoporosis and certain parameters of bone microarchitecture. The specific lipid signature is site-specific and could explain the preservation of bone loss in the mandible.
Team members
Dr Cecile Olejnik (Dentist), Dr. Xavier Coutel (Dentist), Jérôme Delattre (engineer), Nicolas Bertheaume (technician), Alexandrine During (MCF)